COATING PRODUCTS

Our HYDAK® hydrophilic coating technology offers industry-leading performance in lubricity, durability, and particulates.
HYDAK Has Flexible Options For Your Requirements

Only a few microns thick and easily mold to any device substrate.

Bi-laminar coating platform used to covalently bond the coating to the substrate material.

Methods include: ethylene oxide (EtO), gamma and e-beam.

Coatings apply to nearly all polymerics, metals, thin films & silicone.
UV and Thermal Coating Technologies
 |
 |
|
| Ultraviolet (UV) Lighting System | Type of Curing Machine | Heat via Oven |
| Neurovascular, Cardiovascular, Peripheral Vascular | Market Applications | Neurovascular, Cardiovascular, Peripheral Vascular, Ophthalmology |
| Yes | Bi-laminar Platform | Yes |
| Ethylene Oxide (EtO), E-Beam and Gamma | Sterilization Methods | Ethylene Oxide (EtO), E-Beam and Gamma |
| Outer Diameter (OD) | Inner Diameter (ID) vs Outer Diameter (OD) Coating Application | Inner Diameter (ID) & Outer Diameter (OD) |
White paper
Learn More About the Difference Between Our HYDAK® UV and Thermal Coatings.

OUR SERVICES PROCESS
Substrates We Coat





Polymers
Our coatings can be applied to almost all polymerics. If you do not see your substrate listed below, please reach out.
- Nylon/Polyamide
- PEBAX
- Polyurethane
- PEEK
- Chronoprene
- Tecothane
- Tecoflex
- Aesno
- Polypropylene
- Polyethylene
- Hydrophilic films
- Silicone
- …and many more!

Metals
- Stainless Steel
- Nitinol
- Platinum
- Gold
- Nickel
- Titanium

Films
Biocoat HYDAK technology allows for the coating of hydrophilic films used in medical devices. This capability allows for the processing of:
- Implant Delivery Devices
- Wettable IVDs & Microfluids

Plasma
To support the development of cutting-edge medical devices, Biocoat has added dedicated in-house plasma capabilities. The use of plasma treatment supports the coating process by:
- Cleaning the surface to allow for maximum coating adhesion
- Functionalizing the surface to allow to enhance coating lubricity or durability performance
- Altering the surface to allow for unique coating enhancements
HYDAK Performance
By definition, hydrophilic coatings are lubricious and will reduce the amount of friction that is required to maneuver a medical device through the body. Internal research at Biocoat has shown that devices coated with our hydrophilic coatings will routinely reduce the friction force by approximately 95% when compared to an uncoated device.
Due to the many twists and turns during insertion of a device, the coating needs to maintain the same level of performance throughout the entire procedure. This ensures that the coating will remain effective for the duration of the device’s use and still allow for a smooth recovery of the device once the procedure is complete.
Particulates are a relatively new measure of coating performance that is being driven by various regulatory agencies to understand what types of foreign materials are being introduced to the body and what their long-term impacts are to the patient.
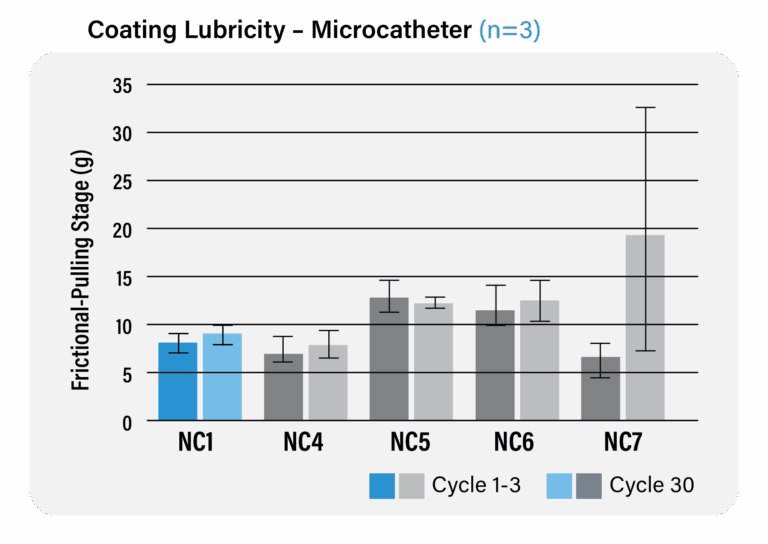
– In this chart, the Biocoat R&D team purchased seven (7) different commercially available neurovascular microcatheters, two (2) of which were coated with Biocoat HYDAK® technology. The Biocoat team measured both the lubricity and durability of each of the microcatheters in an effort to determine where HYDAK® stands in relation to industry competitors. Our testing proves that HYDAK® exhibits best-in-class lubricity and durability results.
– The chart also shows the performance of Biocoat HYDAK UV coating performance in comparison to the seven (7) commercially available microcatheters.
– Data on file.