Authors: Dan Hendrickson, Project Development Engineer, Sarah Libby, Project Development Engineer, and Spencer Bass, R&D Engineer.
Introduction
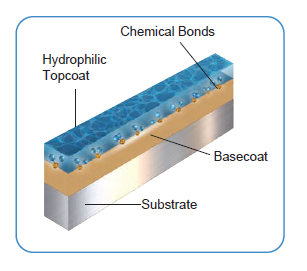
A hydrophilic coating is a polymeric surface treatment engineered to absorb water and significantly reduce the coefficient of friction between a medical device and biological tissue. These coatings are widely utilized on interventional medical devices (especially, Neurovascular, Cardiovascular and Peripheral Vascular devices) , such as catheters, guidewires, and delivery systems to facilitate trackability, minimize insertion force, and reduce tissue trauma.
The functional integrity of a hydrophilic coating is highly dependent on its ability to form a uniform, durable bond with the underlying substrate. Surface cleanliness is a critical, yet frequently overlooked, determinant of coating adhesion and overall coating performance. Organic residues, particulates, oxidation layers, processing aids, and even trace contaminants introduced during manufacturing, assembly, or handling can significantly lower surface energy and disrupt coating-substrate interactions at the molecular level.
A leading cause of adhesion failure observed during feasibility and production runs is surface contamination. These contaminants compromise coating uniformity, increase the risk of delamination, and can result in nonconforming product.
This article outlines the mechanisms by which contamination impacts coating adhesion and provides strategies for maintaining substrate cleanliness throughout the device manufacturing process. Robust surface preparation is essential for ensuring coating durability, minimizing particulate generation, and ultimately enabling safe, high-performance medical devices.
Every Component Matters: Clean from the Start
Device performance begins at the component level. Every material or part used in a medical device should be properly cleaned either in-house or verified through documentation and QA protocols from suppliers. Mold release agents, silicone oils, particulates, and lubricant residues are common on components directly from manufacturing and must be removed before device assembly.
- Recommendation: Implement a formal inspection and cleaning process for all incoming components.
- Supplier Engagement: Work with suppliers to ensure their cleaning processes meet your standards or perform additional cleaning in-house as needed.
- Clean at Every Stage: Clean each device component as you assemble, ensuring new parts don’t introduce contaminants.
Building in a Clean Environment
Ideally, during early development, devices should be assembled in an ISO-certified clean room
to prevent airborne and surface contaminants from affecting sensitive surfaces.


When a Cleanroom is not Available, Alternative Precautions Should be Implemented:
- Extensive Pre-Assembly Cleaning: Use 99% IPA (isopropyl alcohol), solvents, plasma treatment or detergent-based cleaning followed by deionized water rinses and thorough drying with lint free cloth.
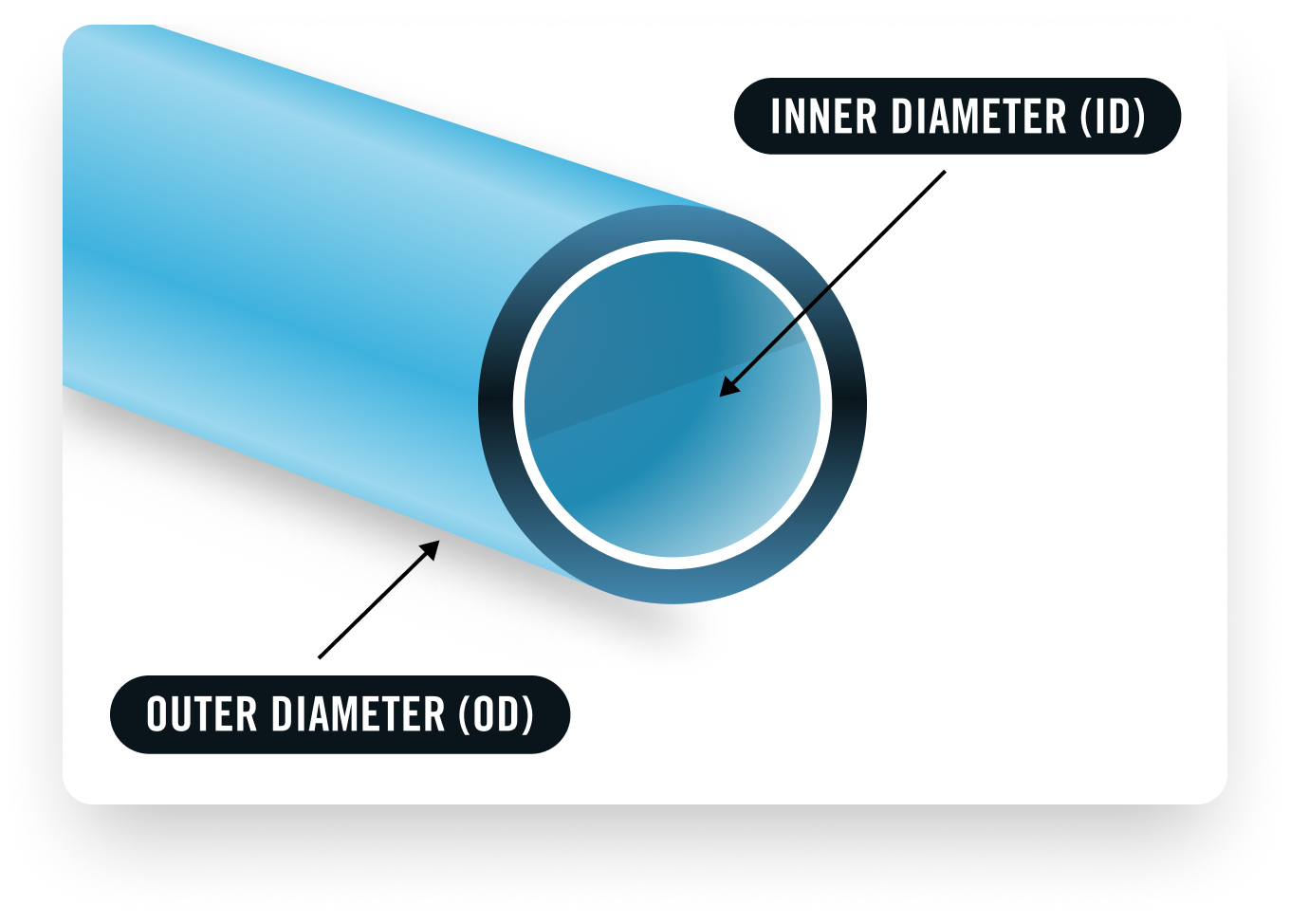
- Air Blow Components: Use filtered, oil-free, dry air to blow out internal diameters (ID) and outer diameter (OD). Also, clean any tubes or hoops used for shipping your device.
- Clean Surfaces: If you place devices down on tables, always use lint-free cloths or clean trays. If a device is directly placed on a table surface, ensure it has been pre-cleaned with IPA and dried with lint free cloths.
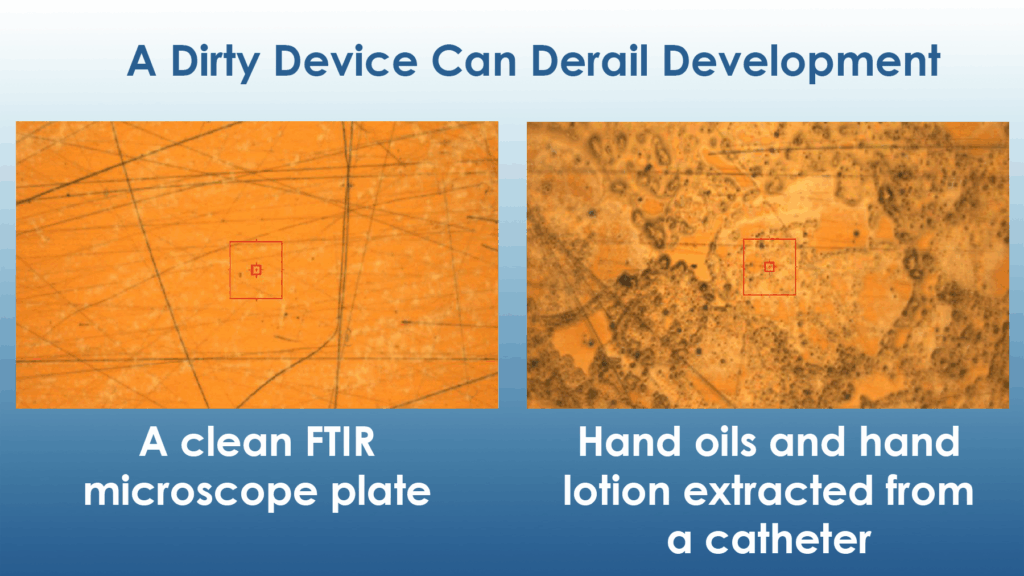
- Wear Gloves Wiped with IPA: Never handle clean devices or components with bare hands. Hand lotion and skin oils can be transferred from your hands to the device. Wiping your gloves with IPA removes particulates and oils that can accumulate when touching gloves with bare hands during the donning process.
- Avoid Silicone Oils and be Aware of Production Mold Releases: These are among the most problematic contaminants, known to cause severe coating defects. Silicone oils may lead to adhesion issues and can easily transfer to other devices and surfaces, potentially causing further complications even when not actively utilized. Additionally, contaminants embedded in substrates can surface under heat or pressure during manufacturing processes like extrusion, reflow, annealing, or coating cures.
Common Contaminants and Their Impact on Coating Adhesion
Below is a chart summarizing common types of contamination, their observed impact on coating adhesion, and recommended cause/source cleaning methods:
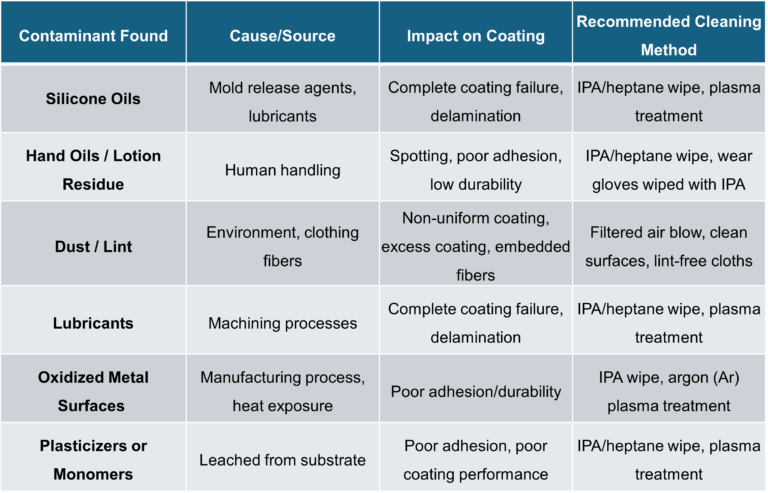
Common Contaminants and Their Impact on Coating Adhesion
How Biocoat Cleans Your Devices
At Biocoat, your devices are managed exclusively in a class 7, ISO 13485 cleanroom. Surfaces are sanitized before packaging is opened, and devices are only touched with IPA- wiped gloves.
Standard practice is to provide five thorough wipes with an IPA-soaked cloth, followed by an ionized air spray. If your device necessitates further cleaning, we provide additional rounds of IPA wiping, heptane wiping, and plasma treatment to ensure optimal cleanliness.
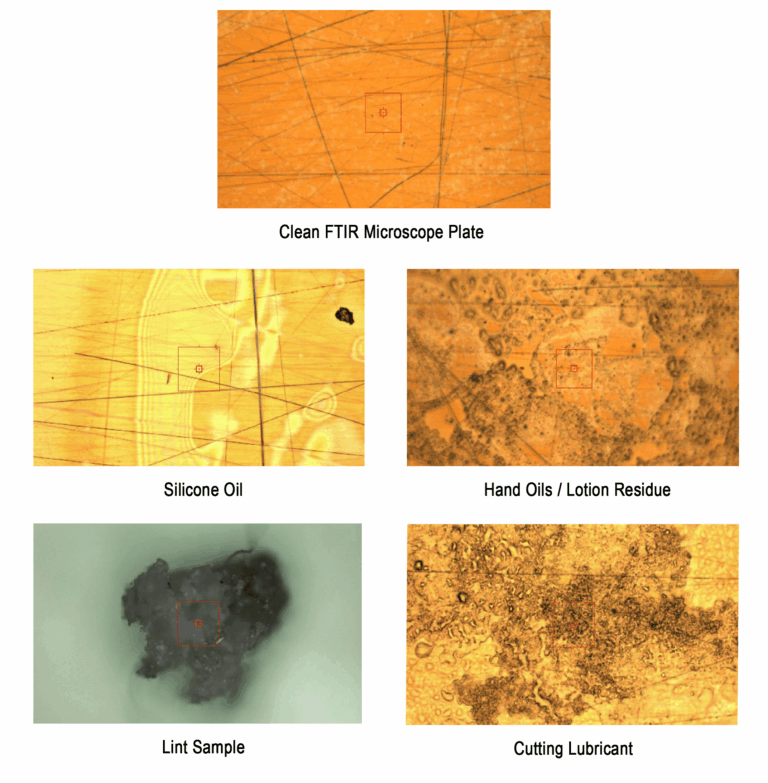
One additional service that we offer, when engaged early in the development process, is FTIR (Fourier Transform Infrared Spectroscopy). This analytical technique enables the early detection of residual processing aids. Identifying these issues at an initial stage allows clients to address them proactively, thereby minimizing potential obstacles and streamlining the path to commercialization.
Conclusion
Surface preparation is one of the most critically and often overlooked aspects of medical device manufacturing. While Biocoat takes extra precautions and lightly cleans your devices upon arrival at our facility, this preliminary cleaning is not sufficient to remove contaminants and particulates from device manufacturing. Therefore, it is critical that the device manufacturer thoroughly cleans the device before it is prepared for a hydrophilic coating. This thorough cleaning ensures the surface is free from oils, particulates, and chemical residues is vital for product success. Whether developing in a certified clean room or a carefully controlled workspace, rigorous cleaning protocols can mean the difference between a high-performing medical device and one that could encounter delays in development or manufacturing.