Biocoat Announces Formation of Surmodics Services & Technologies Division Following Acquisition and Divestiture Transactions.
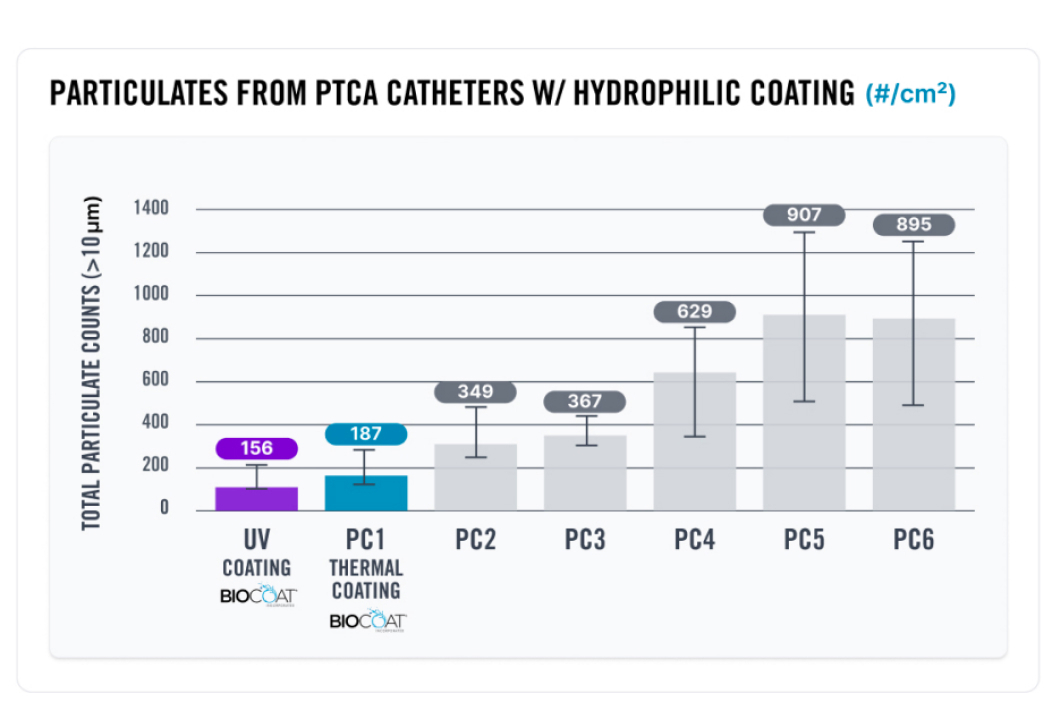
Our hydrophilic coatings provide best-in-class performance with the industry’s lowest particulate counts. Our flexible service model allows you to coat your materials at your production facility or our Contract Coating Services Unit. Your medical device is specifically designed to achieve its intended goal, and our coatings allow your device to be easily maneuverable and highly durable to optimize vascular access and minimize procedure time.
At Biocoat, we are focused on researching, developing, manufacturing, and applying the best coatings in the market. By becoming an extension of your team, we are fully dedicated to providing you with a best-in-class experience at every stage of your product’s development and commercial lifecycle.



Our coatings can be applied to the majority of substrate materials used in peripheral vascular device designs. Our unique coating methods have been successfully used in many applications where other options did not meet the design criteria.

Our coatings can be applied to the majority of substrate materials used in peripheral vascular device designs. Our unique coating methods have been successfully used in many applications where other options did not meet the design criteria.
At Biocoat, our HYDAK® coatings offer best-in-class performance in lubricity, durability, and particulates. Our coatings give you a choice to cure the coatings using either heat or Ultraviolet (UV) light. The following charts show the results of a series of tests that the Biocoat Research & Development team completed to test the friction, durability, and particulates on commercially available microcatheters to evaluate how our coatings perform compared to other coatings on the market.



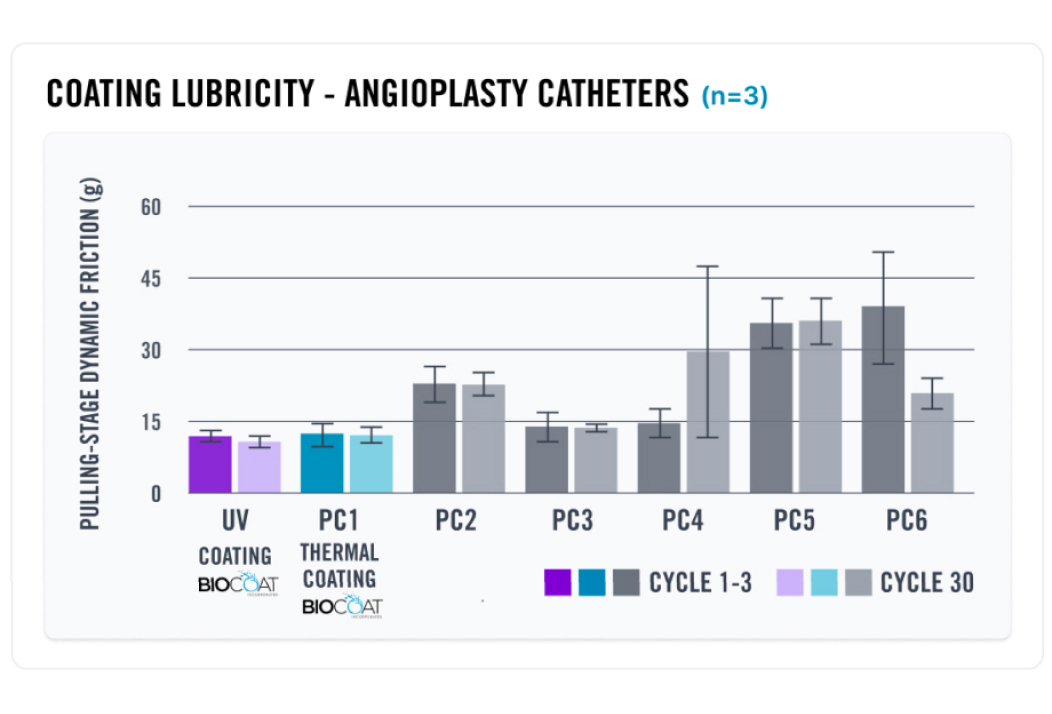
In this chart, the Biocoat R&D team purchased six (6) different commercially available angioplasty catheters, one (1) coated with Biocoat’s HYDAK® Thermal technology. The chart also shows Biocoat’s HYDAK® UV coating performance compared to the six (6) commercially available microcatheters. The Biocoat team measured both lubricity and durability of each catheter to determine where HYDAK® stands in relation to industry competitors. Our testing proves that HYDAK® exhibits best-in-class lubricity and durability results.

Our team of engineers and coating experts would be glad to have a discussion about your project and its requirements.
A major portion of Biocoat’s success has been attributed to our commitment to delivering a high-touch customer service experience. Our coating experts are ready to assist you with the development of a coating that is specifically designed to match your project’s requirements.
The latest news, articles, and resources, sent to your inbox.
