As global attention intensifies on the risks associated with per- and polyfluoroalkyl substances (PFAS), the medical device industry is facing increasing regulatory pressure to eliminate their use. PFAS have historically been used for their non-stick and durable properties. However, as regulatory bodies push for safer, more sustainable alternatives, medical device companies are seeking innovative solutions to meet these demands without compromising product performance. With proposals to limit or ban PFAS use in medical applications in the coming years, manufacturers are racing to adopt alternatives that do not sacrifice quality or functionality.
Biocoat’s Non-PFAS Lumen Coating Method
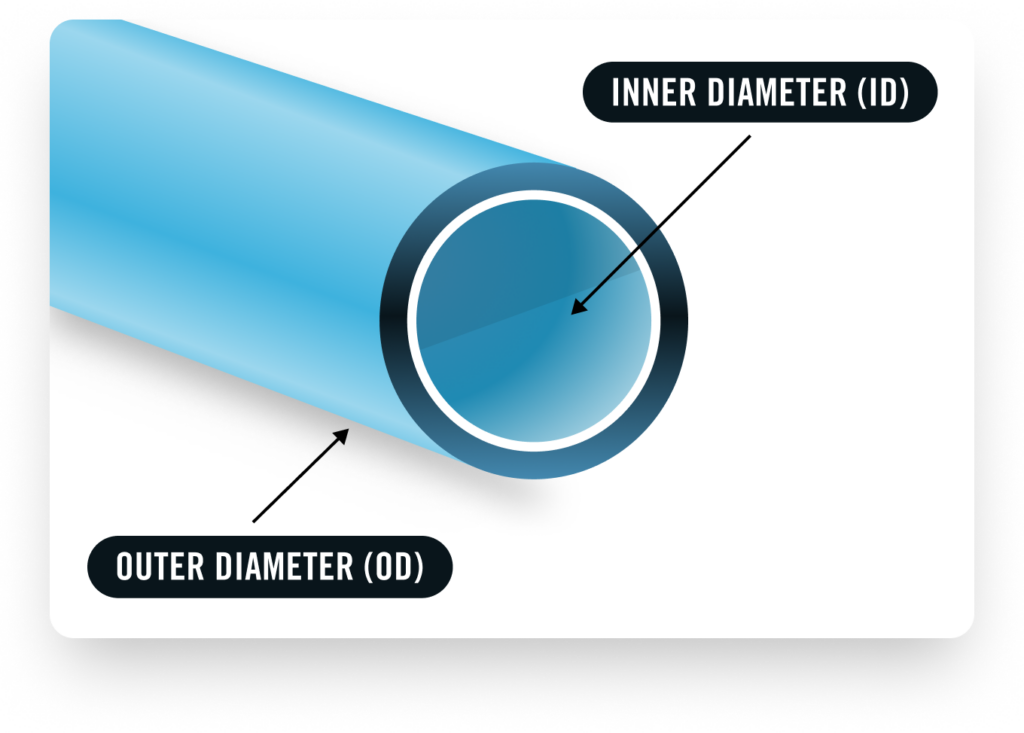
Biocoat, a leading provider of hydrophilic coatings and coating services for medical devices, has been at the forefront of this challenge. The company recently announced that it has secured a patent for its innovative “Non-PFAS Lumen Coating Method and Apparatus”, a thermal cured hydrophilic coating technology specifically designed for the inner diameters (lumen) of medical devices like catheters.
This patented technology marks a significant advancement for the minimally invasive medical device sector, as it not only eliminates PFAS but also offers superior performance benefits. Hydrophilic coatings, known for their lubricity and ability to reduce friction, are critical in enhancing the trackability and maneuverability of devices such as guidewires, catheters, and other minimally invasive products. Biocoat’s non-PFAS hydrophilic coating provides a safe alternative, without compromising these key performance attributes.
The addition of this patent adds to Biocoat’s PFAS Free portfolio which already included the ability to coat directly to metals such as nitinol and stainless steel. This direct to metal capability allows manufactures to eliminate tie-layers, increasing efficiency and creating a thinner coating.
Lubricity and Flexibility for Enhanced Medical Devices
Biocoat’s new coating technology is characterized by exceptional lubricity, which allows medical devices to navigate through narrow or tortuous pathways in the body with minimal resistance. This reduces the potential for tissue damage and can improve efficiency. The lubricious nature of the coating, combined with its durability, ensures that devices maintain their performance throughout the procedures.
In addition to its lubricity, Biocoat’s non-PFAS coating offers impressive flexibility. This is particularly beneficial for devices such as catheters, which require both flexibility and durability to perform effectively in challenging anatomical environments. The coating can withstand the rigors of multiple flexions and stresses, ensuring the longevity of the device.
Positive Innovation in Medical Devices
As the medical device industry braces for upcoming PFAS regulations, Biocoat’s patented non-PFAS lumen coating method stands out as a timely and innovative solution. It not only aligns with the global movement towards sustainability but also ensures that medical devices continue to meet the high-performance standards required for life-saving procedures. With its proven track record of 30 years in hydrophilic coatings, Biocoat is once again leading the way with safe, effective technology for the healthcare industry.
To learn more about Biocoat and their innovations in hydrophilic coatings, visit www.biocoat.com or stop by booth #2828 at MD&M Minneapolis 2024.